
Coronavirus vaccine: How will poorer countries get a fair shot?
It is an unseasonably cold October day in Johannesburg, and Robyn Porteous is following a handful of others up the stairs of a clinic at the Reproductive Health and HIV Institute at the University of the Witwatersrand (Wits).
“I think they have you do this just to prove you don’t have COVID,” quips the 32-year-old digital marketer, as she passes another landing. Three more flights of stairs to go, not that the marathon runner is showing any signs of strain.
“Ventilation,” Porteous explains. The drafts travelling through the windows reduce the risk of transmission for airborne infections such as the novel coronavirus.
South Africa has just come through the country’s first wave of COVID-19. A television in the waiting room plays a local news report featuring the country’s health minister, Zweli Mkhize, speaking about the latest cases. A caption below his image reads: “Mkhize warns against complacency.”
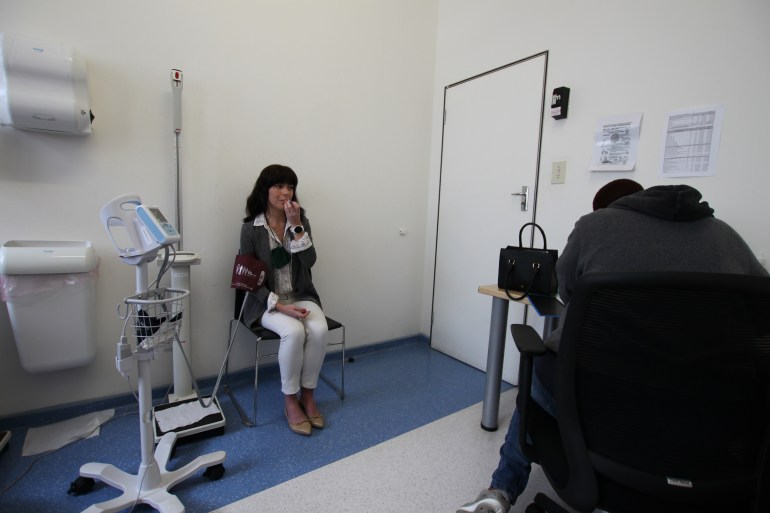
Back in June, Porteous had a heated Twitter debate in defence of COVID-19 vaccine trials in South Africa. It eventually brought her to this very room two months later – where she took her first injection as a volunteer in the AstraZeneca COVID-19 vaccine trial, one of four currently ongoing in the country.
“It was as much like a rise to a challenge as it was just wanting to kind of do something,” she says of her decision to join the study. “You feel really helpless in a pandemic. I can’t donate endless amounts of money but can hopefully donate my body to some kind of scientific research that matters.”
Porteous will now be one of about 2,000 people in the study, which follows an earlier UK trial study that found the vaccine was safe and showed promise in helping the body mount a possible defence to COVID-19.
Vaccine nationalism: Many rich countries claim the ‘lion’s share’
More than four dozen potential COVID-19 vaccines are in human clinical trials, according to the World Health Organization (WHO). Four are being tested in South Africa.
But before any of these jabs have been proven to work, there is another looming roadblock: Some parties, including the United States, European Union and the United Kingdom, are staking their claim to what Oxfam senior policy adviser Mohga Kamal-Yanni calls the “lion’s share” of doses.
A recent analysis by Duke University found that countries have already confirmed purchases for 3.8 billion doses and further 5 billion doses were under negotiation or had been reserved as of late October. Of course, not all experimental immunisations will successfully make it through clinical trials. The US, followed by the EU and India, have so far secured the largest number of potential doses, according to the report.
In September, US Republican Senator Thom Tillis introduced the America First Vaccine Act. If signed into law, the act would prohibit the export of any COVID-19 vaccine developed using government funding until firms had met US demand for it.
“Once that vaccine is developed, Americans should get the vaccine first, before it goes to other countries … ensuring that they receive a return on their investment,” Tillis said in a statement.
 A medical worker talks to volunteers as they wait to receive an injection during South Africa’s first human clinical trial for a potential COVID-19 vaccine in Soweto in June [Reuters/Siphiwe Sibeko]
A medical worker talks to volunteers as they wait to receive an injection during South Africa’s first human clinical trial for a potential COVID-19 vaccine in Soweto in June [Reuters/Siphiwe Sibeko]The US government has invested at least $11bn in COVID-19 vaccine development, according to US consumer advocacy organisation Public Citizen.
The brand of vaccine nationalism behind Tillis’ proposed legislation is not new. During the 2009 H1N1 flu outbreak, high-income countries able to produce vaccines refused to export them until their domestic needs were met, researchers wrote in 2019 in the healthcare journal The Milbank Quarterly.
For a new pandemic, COVID-19 brings with it decidedly old problems when it comes to vaccines – and the world is looking to a decades’ old solution to help.
COVAX: Subsidies and solidarity
In April, the public-private vaccine partnership GAVI launched the COVAX initiative. COVAX aims to pool nations’ purchasing power – and donor funding – to secure a minimum number of affordable vaccines for participating countries through what is called an advanced market commitment. So far, COVAX has secured a possible 700 million potential doses of COVID-19 vaccines, outpacing even the UK, Japan and Canada, according to Duke University.
As part of COVAX, poor countries will pay a subsidised price of up to $4 for a two-dose vaccine. Initially, COVAX promised free vaccines to low-income countries, but a September decision by GAVI’s board opted to introduce a cost-sharing plan with countries. Still, GAVI says there is some flexibility in this requirement and that countries can make a case for why they cannot afford the discounted prices.
Middle and high-income countries will pay in full for stocks procured through COVAX. Nations that may have already reserved supplies of some vaccines can also opt to use COVAX to buy doses they were not able to secure through bilateral deals.
The US has already said it will not join COVAX.
Ultimately, COVAX hopes to procure two billion vaccine doses by the end of 2021 and guarantee participating countries enough vaccines to immunise up to 20 per cent of their populations. As of 19 October, 82 countries had signed legally binding contracts to join the initiative, which has already raised more than $2bn in funding.
COVAX draws on GAVI’s success with a similar 2005 initiative to introduce a pneumococcal vaccine. Pneumococcal disease is a bacterial infection that can lead to fatal conditions such as meningitis and pneumonia; hundreds of thousands of children died from it in lower-income countries before this initiative.
But 15 years later, will COVAX be enough to guarantee countries a fair shot at a future COVID-19 vaccine? The mechanism may not be perfect, say some, but it might be many countries’ best – and only – option.
Vaccine scepticism: ‘People build up what they think a trial is’
Back at the vaccine trial at Wits, a doctor in dark blue scrubs enters the waiting room.
“Number 1403?” he calls out. A young man looks up from the phone he has been scrolling through and rises to meet him before the pair head off down another hallway.
Porteous, the daughter of healthcare workers, grew up watching VHS tapes of surgeries. Medicine, she says, was dinner table talk. Still, it doesn’t mean her parents were thrilled that their daughter signed up for a clinical trial.
“My parents, being medical, understood the need for the trial, but they were like, ‘We don’t necessarily want our daughter doing it’,” she says. “But once I went through the science behind it with them, explaining, it’s not like they’re injecting you with COVID, they were fine with it.”
She adds: “Everyone kind of hears ‘COVID vaccine trial’ and their mind goes to the worst possible result.”
She reflects on the Twitter debate that brought her to the study. Other South African Twitter users had expressed concern that an experimental COVID-19 vaccine was being trialled in Africa. But it had already been proven safe in human trials in the UK, Porteous explained in a tweet.
“So why don’t you volunteer?” came the response from one Twitter user.
 Vaccine trial participant Robyn Porteous has her blood drawn [Garret Barnwell/Al Jazeera]
Vaccine trial participant Robyn Porteous has her blood drawn [Garret Barnwell/Al Jazeera]“What surprised me a lot from being online and on Twitter was the perception of this vaccine trial,” Porteous tells Al Jazeera. “From the get-go, people were convinced that there were these nefarious intentions involved.”
“I felt like that made it even more important for me to take part so I could say, ‘Look, I volunteered and I’m okay… nothing was done against my will,’” she explains.
“Number 1389,” a nurse calls out in the clinic’s waiting room. Porteous, who has been leaning against a doorway, steps forward and follows her into a small office.
“People build up what they think [a vaccine trial] is and it’s almost like the truth can’t get through that … and that’s a bit of a problem,” Porteous says before she exits.
A history of medical colonialism
But those who are sceptical can point to a long history of medical colonialism in Africa – some of it in the not-so-distant past.
During the German colonisation of Namibia in the late 1800s and early 20th century, for example, the Herero and Nama people were forced into concentration camps. In one camp, a German doctor injected prisoners with arsenic and opium in a failed bid to study vitamin C deficiency.
German professor of medicine, and future Nazi, Eugen Fischer visited the country during this time to collect what is estimated to be hundreds of skulls and skeletons of murdered Namibians for “research”, writes University of Namibia Professor Vilho Amukwaya Shigwedha in a 2018 book. Germany has begun to repatriate these remains.
Karsten Noko is a Zimbabwean lawyer and medical humanitarian worker who has written about the ways in which colonialism has shaped medicine in Africa.
 In a photo from 2015, a nurse administers an injection on the first day of the Ebola vaccine study being conducted at Redemption Hospital in Monrovia, Liberia [File: Getty Images]
In a photo from 2015, a nurse administers an injection on the first day of the Ebola vaccine study being conducted at Redemption Hospital in Monrovia, Liberia [File: Getty Images]In the 20th and 21st century, medical colonialism may look quite different but it still betrays what Noko says are double-standards when it comes to medical ethics. What is done to African bodies, he tells Al Jazeera, is not always what would be done to those in the Global North.
In 1996, pharmaceutical company Pfizer tested an experimental drug on 200 Nigerian children without parental consent. Community members eventually sued Pfizer and settled out of court.
Almost a decade later, blood samples taken from Ebola patients during West Africa’s 2014 epidemic were subsequently relocated in secret to laboratories as far away as the UK without patient consent, the Telegraph newspaper discovered.
Noko says that incidents such as these – coupled with a lack of transparency from pharmaceutical companies around trials, medicine pricing and patents – fuels distrust. Meanwhile, there remains a dire need for diverse clinical trial data to ensure that medicines and vaccines work for everyone, including Africans.
“We understand that if people are not willing to consent to clinical trials, then we do not get drugs. That’s not what we want,” he explains. “But what I do think we should be calling for … is much more transparency about what happens and much more protection from states.”
Today, clinical trials in countries like South Africa, Kenya and Uganda are subject to heavy regulatory oversight and must meet stringent national and international standards that ensure that patients are protected, that communities have a say in clinical trials and, in some places, that African scientists play leading roles in local research.
 Protesters demonstrate against COVID vaccine testing on Africans, at Wits University [Reuters/Siphiwe Sibeko]
Protesters demonstrate against COVID vaccine testing on Africans, at Wits University [Reuters/Siphiwe Sibeko]Dr Kathy Mngadi is a clinical research site leader with The Aurum Institute and helps head major HIV vaccine trials in South Africa. As a researcher, she says she understands why some people might distrust and misunderstand research studies.
“I think one of the remnants of apartheid and colonialism has been a deep mistrust by the majority of the population of not just past but even current government structures,” she says.
Allegations of government corruption related to the country’s COVID-19 response and the poor quality of public health services in some areas only add to this distrust, she explains.
But HIV clinical trials like the ones Mngadi leads have also helped spearhead a solution.
HIV activists and researchers have largely led the global push for meaningful community participation in trials. This means that representatives from communities that could be affected by trials or who live in the surrounding area should be involved in the design, running and post-trial follow-up of research trials.
Today, in large part thanks to HIV activists and scientists, community participation is part of international best practice when it comes to all human clinical trials.
“We need to involve the community from the beginning so that we see research is not something that is done to communities, but rather that it’s done with communities,” Mngadi explains.
At least three of the major COVID-19 vaccine trials in South Africa are using existing HIV research sites, which have long-standing community advisory boards comprising community leaders and representatives who ensure community interests are safeguarded during the trial process. Additionally, independent experts monitor trial data and can call for studies to be halted if anything goes wrong.
Still, Mngadi knows scientists have a long way to go when it comes to helping the public understand the ways clinical trials work to ensure volunteers are safe and that communities benefit from medical research conducted where they live.
“When I see a social media posting or article in South Africa about people being used as ‘guinea pigs’ it becomes quite clear to me that people are not aware of all of these layers of protection that are put in place during clinical trials,” she says.
“You can’t blame them because they don’t work in clinical research, but I do think it’s important for researchers to continuously get that message out.”
COVAX round one: Lessons from pneumococcal disease
About 30km south of the Wits clinic in Johannesburg, the sign for Soweto’s Chris Hani Baragwanath Academic Hospital stretches across five lanes of traffic entering and leaving the facility – a testament to how large and busy the township hospital is.
Wits Vaccinology Professor Shabir Madhi’s office sits on the 11th floor of the nurses’ residence at the hospital. From the floor-to-ceiling windows, Soweto stretches out in a patchwork of red, white and sandy-coloured roofs.
By 2005, more than 100,000 children under the age of five were being admitted annually to facilities just like Chris Hani Baragwanath in South Africa with pneumococcal disease.
Globally, hundreds of thousands of children under the age of five were dying annually from the disease. Almost six out of 10 such deaths were in Africa by 2008, according to the WHO.
A vaccine to prevent pneumococcal disease had debuted in the US in 2000 but, eight years later, was still too expensive for countries in the Global South.
Developing a new vaccine is a risky business financially. It can take decades and millions of dollars to produce a new immunisation, according to the US Center for Global Development. If poorer countries cannot afford to pay, firms do not invest in producing vaccines because they cannot be assured of a return on investments.
 Minibus taxis transport commuters to work, in Soweto, Johannesburg [Reuters/Siphiwe Sibeko]
Minibus taxis transport commuters to work, in Soweto, Johannesburg [Reuters/Siphiwe Sibeko]In the early 2000s, global health experts came up with an idea to overcome this – an advance market commitment. The concept seems simple: If poorer countries pooled their buying power, they could assure would-be vaccine makers of their ability to pay for large volumes of doses upfront.
Vaccine-makers would be guaranteed a return on investment and, in exchange, would promise immunisations at a pre-agreed price for a certain period – much like COVAX.
Activists have long known that markets do not work to provide medicine for those who need it most. That is why in 2016 a high-level United Nations panel of experts proposed advance market commitments as one of a series of innovative funding approaches to ensure that the cost of making a medicine or vaccine did not necessarily determine its price.
In 2007, GAVI piloted the idea with an advance market commitment for the pneumococcal vaccine. As part of the agreement, companies committed to providing doses for a fraction of what countries would typically pay – $3.50 instead of $50 per dose, according to 2008 prices.
Previously, low-income countries had to wait decades before vaccines that were available in the Global North reached their shores, but GAVI’s advanced market commitment changed that, says Professor Madhi.
“With the pneumococcal vaccine, that 20-year lag time was reduced to 10. And it’s all about the funding model,” explains Madhi, who is also the lead researcher on two of the COVID-19 vaccines being tested in the country as of late October through the university’s Vaccines and Infectious Diseases Analytics (VIDA) unit.
Less than a decade after GAVI’s first advance market commitment launched, 54 countries from Afghanistan to Zimbabwe were able to roll out the pneumococcal vaccine, an independent review by the Boston Consulting Group (BCG) found. And by 2015, the deal had saved the lives of almost 300,000 children.
 Wits Professor of Vaccinology Shabir Madhi at Chris Hani Baragwanath hospital in Soweto [Luca Sola / AFP]
Wits Professor of Vaccinology Shabir Madhi at Chris Hani Baragwanath hospital in Soweto [Luca Sola / AFP]Because South Africa is considered a middle-income country, it did not qualify to get cheaper pneumococcal vaccines through GAVI – and it will not get heavily subsidised COVID-19 vaccines if it signs onto COVAX.
Despite this – and largely through its own efforts – in 2009, South Africa became the first African country to introduce vaccines to prevent both pneumococcal disease and rotavirus, which causes diarrhoeal disease. The move was in part because of research conducted by Madhi and his team at the Soweto hospital, including one of the first pneumococcal vaccine studies.
Personal and public health value: The rotavirus vaccine
The earliest human clinical trials into any new vaccine will test it among a small group of people to make sure a jab is safe but not if it works. Safety data such as this is more-or-less universal from one country to the next, Madhi explains. But genetics, living conditions, and how much of a given disease is circulating in a population can all play a role in how well a vaccine works from one population to the next.
Differences like these may explain why a 2010 study published in the New England Medical Journal by Madhi found that the rotavirus vaccine aimed at protecting children from diarrhoeal disease was less effective in Malawian infants than in South African children.
Why? Madhi and his team believe that it is because of the higher prevalence of rotavirus in Malawi. Babies there are more exposed to the virus by the time they reach five months and develop some natural immunity to it. Adding a vaccine only boosted this existing immunity a little.
In contrast, most South African infants have no pre-existing immunity to rotavirus because there is less of the virus circulating in the country, meaning they are less likely to be exposed to it as babies. For children like these on an individual level, the vaccine made a bigger impact because they had zero immunity to the virus, to begin with.
But on a population level, the result of this looks different. Madhi and his team found that for every 100 babies vaccinated in Malawi, the country prevents about seven cases of deadly diarrhoeal disease – almost twice as many cases averted by vaccinating the same number of children in South Africa.
Again, it came down to how prevalent rotavirus was in Malawi. With more of the virus circulating there, children were more likely to die from the disease than their South African peers – despite the fact that Malawian children might have picked up some immunity to the virus as babies.
Because Malawian children’s odds of dying from rotavirus were so much greater than those experienced by South African children, even a slight increase in protection against rotavirus made a big dent in deaths on a population level, researchers explain in the 2010 study.
“The public health value of that vaccine, despite the lower efficacy in Malawi, is actually greater in Malawi,” Madhi explains, “because Malawi has just got much more of the disease occurring.”
 A health worker talks to a COVID-19 vaccine trial volunteer before he is tested at the Wits clinic [Reuters/Siphiwe Sibeko]
A health worker talks to a COVID-19 vaccine trial volunteer before he is tested at the Wits clinic [Reuters/Siphiwe Sibeko]Eventually, similar work by Madhi in South Africa provided policymakers and treasury officials with the local data to show that not only would pneumococcal and rotavirus vaccines save lives in the country, but also that they would be cost-effective, even at the pneumococcal vaccine’s price of $20 in 2009.
Madhi received a lifetime achievement award from the South African Medical Research Council for his work. At the ceremony, he thanked the parents of the children who had participated in that first pneumococcal vaccine trial in Soweto.
He told the South African Medical Journal in 2013: “There are very few places in the world where you’ll find parents willing to take on the risk of having their kids participate [in a trial] – based purely on the notion that it will benefit other kids in a future generation.”
Supply constraints, intellectual property: Barriers to global coverage
The world’s first advance market commitment for a vaccine unequivocally sped up access to pneumococcal immunisation, BCG’s 2015 review found. By 2030, it is estimated that the programme will have saved three million lives.
GAVI’s advance market commitment also prompted pharmaceutical companies to tailor the vaccines for low-income settings by, for instance, making them easier to transport. And it proved that a large low-income market would be available even after the advance market commitment ended, the document argues, prompting more investments by firms.
But the project also had limits, the 2015 review acknowledges. For one, it wasn’t designed to increase the number of companies who could make the difficult-to-produce pneumococcal vaccine, which would have bolstered supply and brought down prices faster. And existing makers weren’t always able to fill the demand for the jabs.
To spark more manufacturing, experts suggested in 2015, GAVI could have – for instance – set funds aside specifically to develop capacity among firms in the Global South that could have eventually produced pneumococcal vaccines more cheaply.
Some think COVAX is repeating these mistakes. Like the pneumococcal advance market commitment, COVAX is trying to expand the supply of possible COVID-19 doses by increasing manufacturing. But Kate Elder, senior vaccines policy adviser for Doctors Without Borders’ (MSF) Access Campaign, says that COVAX is not going far enough to demand pharmaceutical companies commit to letting others produce patented vaccines.
“We remain in a model where the pharmaceutical industry has all the control over what volumes of these future vaccines they produce as they did back in the day with pneumococcal vaccines,” she argues.
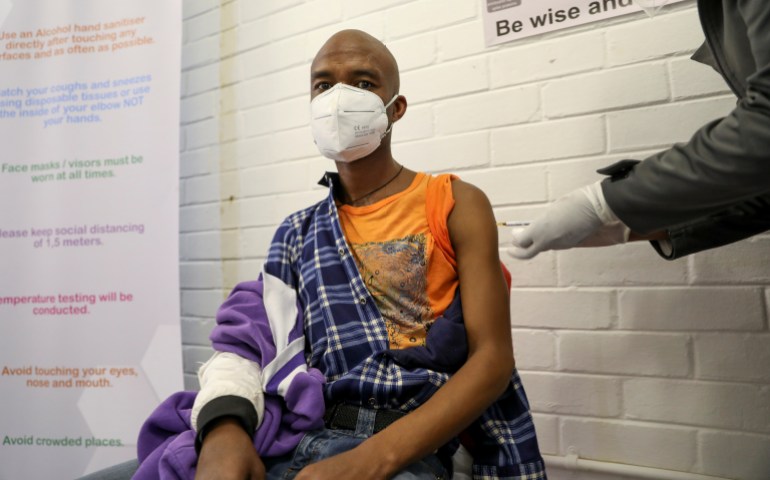 A volunteer receives an injection during a human clinical trial for a potential COVID-19 vaccine, in Soweto in June [Reuters/Siphiwe Sibeko]
A volunteer receives an injection during a human clinical trial for a potential COVID-19 vaccine, in Soweto in June [Reuters/Siphiwe Sibeko]GAVI’s Managing Director of Country Programmes Thabani Maphosa disagrees.
“Intellectual property – in the case of vaccine manufacturing – represents only a part of the relevant expertise required to establish new capacity,” he tells Al Jazeera. “The more challenging aspect involves know-how and high start-up costs as vaccine production requires thousands of manufacturing steps.”
Maphosa says it is not realistic that countries without the current ability to produce vaccines will develop it within just months and in time to meet the world’s immediate need for COVID-19 immunisations.
“Supply constraints – not intellectual property issues – will be the biggest barrier to achieving global coverage of COVID-19 vaccines,” he adds. “COVAX is instead focusing on raising enough cash to incentivise manufacturers to make enough doses to satisfy global demand.”
Still, Elder believes COVAX could do both.
“You can expand manufacturing capacity,” she says, “and [ensure] intellectual property barriers aren’t a hindrance for any manufacturer that has the capacity to produce quality-assured, future COVID-19 vaccines.”
Caught in the middle: Countries not poor enough for subsidies
Madhi agrees that intellectual property is not the most pressing problem when it comes to COVID-19 vaccines.
But he also does not think COVAX is necessarily the best option for South Africa.
“Paradoxically, the COVAX facility doesn’t actually work in favour of South Africa,” Madhi says. “South Africa is categorised as an upper-middle-income country, which basically means that it needs to pay the full price for vaccine procurement and we know what the economy is like.”
South Africa’s treasury was recently forced to re-arrange the national budget to devote a further R21.5bn ($1.3bn) to healthcare amid the current pandemic, and the national treasury department has warned that “a fiscal reckoning looms”.
By some accounts, just a downpayment on vaccines for a country such as South Africa could cost millions. GAVI declined to disclose details about how much countries were asked to pay as deposits on future orders, saying it was up to national governments to disclose this. The South African health department did not respond to requests for comment on how much the country was asked to pay as a COVAX downpayment or whether it has paid.
“It’s going against the global spirit of COVAX, but it might be more cost-effective for South Africa to actually engage directly with manufacturers to get the vaccine at a much more affordable price,” Madhi suggests.
 A mother and her children walk past public toilets at an informal settlement in Kliptown, Soweto. South Africa is considered a middle-income country and will not qualify for cheaper COVID-19 vaccines [Reuters/Siphiwe Sibeko]
A mother and her children walk past public toilets at an informal settlement in Kliptown, Soweto. South Africa is considered a middle-income country and will not qualify for cheaper COVID-19 vaccines [Reuters/Siphiwe Sibeko]But what makes a vaccine cost-effective is how well it works and how much of it might be needed. And, like the pneumococcal vaccine, this may not be the same for all countries.
First waves of COVID-19 infections in countries such as South Africa and India have played out very differently and much less deadly than, for instance, in Spain, Madhi notes.
In Spain, roughly 10 percent of adults had contracted the new coronavirus after the first wave of infections. But in South Africa, this percentage could be as high as 45 percent, Madhi says based on preliminary data from the country’s Western Cape province. Spain had reported 36,257 COVID-19 deaths as of early November – almost double the 19,465 recorded in South Africa.
Scientists still cannot say for sure what kind of immunity COVID-19 survivors are left with after initial infection – or what kind of immunity a future vaccine will confer. But if contracting the virus does offer some protection against future infections, countries such as South Africa might need fewer vaccines than nations such as Spain, Madhi explains.
Only better local data will tell. In the meantime, South African President Cyril Ramaphosa confirmed on November 11 that the country is in talks with several pharmaceutical companies for future vaccines. Two firms may produce COVID-19 vaccines in the country one day, Ramaphosa says.
‘No vaccines in sub-Saharan Africa until the pandemic passes’
Back at the clinic in Johannesburg, a nurse seated in a swivel chair in a small consulting room is taking Porteous’s blood pressure and temperature. She sticks a thermometer in Porteous’s mouth before turning back to face her desk.
“Can you confirm your name and surname?” the nurse asks.
Porteous gestures that she cannot speak with the thermometer still between her lips.
“Oh, right,” says the nurse as the pair begin to chuckle. They wrap up the exam, and Porteous heads back out to the waiting room. It is almost 11am, and staff at the trial are handing out small packets of instant soup and potato chips to those waiting.
Outside, the fog has begun to lift. Porteous hopes that if the vaccine being tested in her trial works, South Africa will get the doses at an affordable price.
“Otherwise, I’m just a mouse in the cage in the cog of the capitalist machine, which is pretty depressing,” she says, only half laughing.
But if South Africa is not able to get a vaccine at all?
“That in itself would undermine what we’re trying to do here because there is a history of African bodies being used as test subjects without benefit to those African bodies,” she says, referring to the continent’s history of colonisation. “If I were to discover that in some way I’d been complicit in reinforcing that narrative, I’d be incredibly upset.”
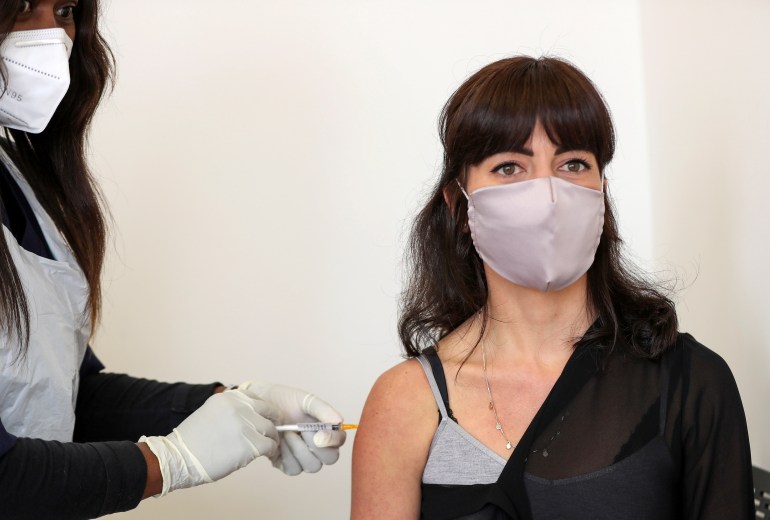 Robyn Porteous is injected with a vaccine as part of a clinical trial [Reuters/Siphiwe Sibeko]
Robyn Porteous is injected with a vaccine as part of a clinical trial [Reuters/Siphiwe Sibeko]Several pharmaceutical companies, including those behind two of the vaccines being tested in South Africa, AstraZeneca and Novavax – along with AstraZeneca’s partner Oxford University – have said they will forgo taking any profits from vaccines sold to low-income countries. Pharmaceutical company GlaxoSmithKline, which is working with the firm Sanofi, says it does not expect to profit from COVID-19 vaccines in this pandemic.
But many activists, including MSF, point out that it is almost impossible to monitor whether companies stand by these no-profit pledges if pharmaceutical companies refuse to disclose how much it takes to make the vaccine itself. How much it costs to make drugs and vaccines is a closely guarded secret in the world of medicine and without this information, activists have been historically handicapped in countering claims that high prices set by firms were needed to recoup investments in research and development.
Without knowing exactly how much pharmaceutical companies spent on researching, developing and producing COVID-19 vaccines, it is impossible for the public to know how much profit they stand to make from them, MSF’s Kate Elder said in a recent statement. It is also impossible to know how much public funding bankrolled the end price.
“Pharmaceutical corporations Sanofi and GlaxoSmithKline must sell their vaccines at-cost and open their books to show the public exactly how much it costs to make the vaccine,” Elder argues. “There is no room for secrets during a pandemic and past experience tells us that we can’t take pharma at their word without data to back up their claims.”
If Novavax’s immunisation is successful, South Africa will receive priority access to vaccines at about $3 a dose from the Serum Institute in India through a deal between Serum and the Bill & Melinda Gates Foundation, Madhi says. Political pressure may lead AstraZeneca to say the same.
$3 a dose is roughly what low-income countries are being asked to pay for immunisations obtained via COVAX and cheaper than what South Africa will pay through the GAVI mechanism.
But for the rest of the world, there are few other immediate options than COVAX.
“The reality is that the majority of countries where 87 percent of the world’s population live, are going to have a very limited supply of vaccines, at least for 2021,” Madhi says.
“It won’t work in every country’s favour, but from a global perspective, the COVAX facility is the one [option] that will ensure timely and reasonably equitable access to and distribution of vaccines,” he continues.
“Outside of the COVAX, all that will end up happening is that we won’t get vaccines in sub-Saharan Africa until the pandemic is past. We have to accept that.”


