President Nana Addo Dankwa Akufo-Addo has justified Ghana’s decision to approve a new malaria vaccine developed by scientists at Oxford University.
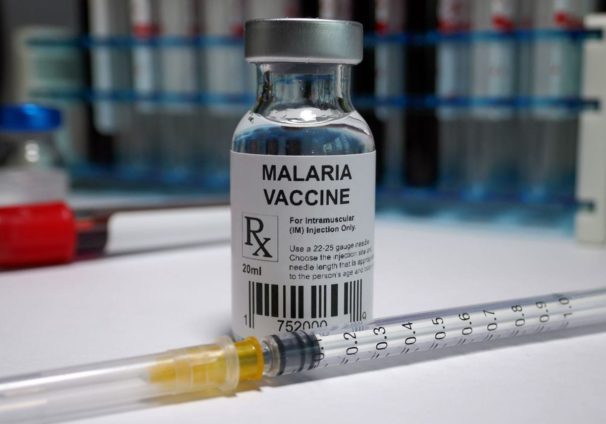
Ghana is the first country to approve the new R21 malaria vaccine, described as a “world-changer” by the scientists who developed it.
Speaking at the inauguration of the DEK Manufacturing Plant in Accra, President Akufo-Addo said the government approved it because the vaccine has proven to be safe.
“The approval was granted following an extensive series of reviews and further peer reviews of the non-clinical and clinical quality parts of the vaccines. The R21 malaria vaccine has been approved for use for the immunisation of children between 5 months and 36 months against malaria,” President Akufo-Addo explained.
The vaccine was developed by Oxford University and manufactured by the Serum Institute of India.
The Food and Drugs Authority (FDA) approved the new R21 malaria vaccine to help fight the disease, a major cause of mortality among children under five in Africa.
The R21 malaria vaccine will be administered to children aged between five to 36 months against malaria caused by Plasmodium Falciparum.
Meanwhile, President Akufo-Addo has cut sod for the construction of DEK Vaccines Limited, a vaccine manufacturing factory, to make Ghana self-sufficient in vaccine production.
With an investment of $122.6 million, the project will witness a significant step towards securing the health of Ghanaians through locally manufactured vaccines that meet global quality standards.